
Want to learn more about the FDA’s next steps on CBD regulations? Don’t miss the Hemp Industry Daily Forum at MJBizCon 2020, presented live Nov. 30 and available on demand.
CBD businesses looking for regulatory clues from U.S. food and drug regulators were watching closely in mid-November, when the U.S. Food and Drug Administration reassured the industry and the public that the science of CBD and other cannabinoids has become a priority.
But the FDA gave little indication that those regulations will be coming soon. Instead, researchers discussing data on how cannabis compounds affect women and men differently repeatedly identified research gaps.
Much of the data that was presented during the conference showed that there are open questions about CBD that will take time to sort out, said Jonathan Havens, head of the cannabis practice group at the Saul Ewing Arnstein & Lehr law firm in Baltimore and Washington DC.

Dr. Douglas Throckmorton, FDA’s deputy director for regulatory programs, explained the agency’s approach to CBD to kick off a conference about cannabinoids and gender.
“It gives FDA … the cover to say, ‘Look, we just don’t have the kind of data that we want in this space in order to proceed expeditiously,’” Havens said.
He pointed out that the November conference looked just at gender differences, not even scratching the surface on research in other populations, such as youths and the elderly.
The bright side for the industry may have been that there weren’t any glaring data against CBD presented, Havens added.
Hemp Industry Daily combed through the breakout panels to find the most important business takeaways from the researchers. Here are eight key takeaways to know about the data the FDA reviewed:
1. Consumers want input on CBD from doctors
CBD is appealing to many because it doesn’t provide the high from THC, so people are curious, said Shari Berman, owner of Canna Healing Consulting in Boston.
Berman told the FDA that she personally pursued the use of cannabis during bouts with cancer, but didn’t receive help from her doctors even after medical marijuana was legalized in her home state of Massachusetts.

Shari Berman
“There is less stigma associated with using hemp CBD than cannabis,” said Berman, who now advocates for other patients.
Most consumers worry about a lack of regulation because of mislabeled and potentially unsafe products, Berman said. Right now, “patients are driving this, not doctors,” she said. Most product labels direct consumers to consult with their doctors, but medical professionals are not trained or allowed to advise patients on how to use CBD.
“Patients want to know how to use (CBD) and want to be able to have conversations with their doctors, but most of the time, doctors shut people down and it makes people nervous about using it,” Berman said.
The way cannabis is scheduled currently inhibits research, Berman said. She called for cannabis training in medical schools and for FDA to involve consumers in the CBD Working Group.
2. FDA is looking at CBD, but that’s not all
FDA is looking at research and data from all cannabinoids, not just CBD. Much of the data presented during the cannabinoids, sex and gender conference was in regard to THC’s effects on anxiety, pain, pregnancy and women’s health.
The agency says there are research gaps on minor cannabinoids as well as other non-cannabinoid molecules such as terpenes.
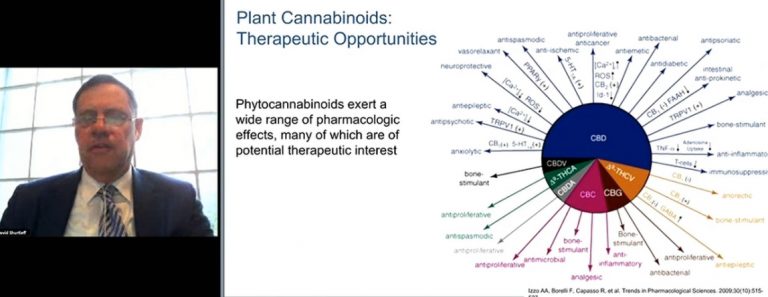
“The focus of research for many years has been on THC, and it’s left significant gaps in understanding the properties of the well over 100 cannabinoids and 120 terpenes within the cannabis plant. Many may have important therapeutic benefits,” said David Shurtleff, deputy director of the National Institute for Health’s National Center for Complementary and Integrative Health.
He said that phytocannabinoids “exert a wide range of pharmacologic effects” that are of therapeutic interest.
He said several NIH institutes have “modest investments in sex and gender in cannabinoid research,” including research focused on the endocannabinoid system and clinical research focused on the therapeutic effects of cannabinoids. Hemp Industry Daily counted at least 11 projects underway.
The FDA is looking at health effects of both marijuana and federally legal hemp, and has separate working groups focused on each.
3. CBD may help with anxiety
Preliminary research results show that CBD is more effective in lowering anxiety than THC and shows more pronounced reduction in female subjects than in male subjects, according to Cinnamon Bidwell, director of the Center for Research and Education Addressing Cannabis and Health at the University of Colorado, Boulder.
Bidwell said women tend to be more anxious than men and respond differently to cannabis. Cannabis products were sourced from Colorado dispensaries and patients were tested both before and after cannabis use.
Bidwell said data collection for the study is ongoing through 2021, and she is looking to expand her research to include a more diverse patient sample.
4. CBD shows efficacy in pain management
According to Dr. Daniel Clauw of the University of Michigan, men and women respond differently to cannabinoids when they are used for pain. Men have a “lower amplifier setting” for pain, Clauw said, while women have 1.5-2 times more chronic pain than men, which originates more from the central nervous system, on average.
Clauw, who teaches medicine, anesthesiology and psychiatry, said that cannabinoids exert potential pain-relieving effects through different mechanisms of action.
CBD, in particular, has peripheral anti-inflammatory effects that may be responsible for pain relief, and might be more effective for nociceptive pain — originating from the periphery such as acute pain, osteoarthritis and rheumatoid arthritis — as well as centralized or nociplastic pain, which originates from the central nervous system.
5. CBD may have applications for managing addiction, psychosis
In studies of patients dealing with cannabis use disorder, Yasmin Hurd, director of the Addiction Institute at Mount Sinai, found that using CBD suggests reduction of cannabis, opioid and alcohol use and cravings and anxiety in heroin addicts — but the evidence is still weak given the small sample sizes.
In relation to psychosis, CBD and THC have been shown to have opposite effects on regional brain activation across a variety of cognitive tasks in healthy individuals.
CBD also reduces the effect of anxiety and cognitive effects elicited by THC, reduces positive symptoms in schizophrenia patients, and appears effective in high-risk patients.
6. Hemp-CBD research on pregnant and nursing women greatly lacking
When it comes to pregnant and nursing women, a lot more is known about the effects of using THC during pregnancy, including craniofacial malformations in mice similar to those caused by fetal alcohol syndrome.
According to Scott Parnell, assistant professor of cell biology and physiology at the University of North Carolina, Chapel Hill, THC and CBD, and particularly synthetic cannabinoids, disturb embryo and fetal development.
He said more epidemiological studies in humans are needed to learn the effects of cannabinoids during other developmental stages, long-term use effects during pregnancy and behavior effects.
Overall, there was no specific data presented on the use of hemp-derived CBD during pregnancy and lactation.
Any data that currently exists for CBD isolate is in regard to use of Epidiolex on animals at high levels, but that would be different compared to doses given to humans and lower-dose, over-the-counter products, according to Dr. Mark Zakowski, anesthesiologist at Cedars-Sinai Medical Center in Los Angeles.
In terms of hemp-derived CBD-specific use, the conclusions are far from solid and unless it’s a clear therapeutic use, pregnant and lactating women are likely not getting any benefit from CBD, so the default is “don’t use it,” said dr. Nathaniel DeNicola, an environmental health expert with the American College of Obstetricians and Gynecologists.
7. Women need to be included in more research
Traditionally, scientific studies have focused on male physiology, and there continues to be a need to remove barriers for women to get into studies, according to Betty Jo Salmeron, staff clinician for NIH’s National Institute on Drug Abuse.
“NIH has ratcheted up the enforcement of studying sex as a biological variable because women were not included in studies,” Salmerson said.
It’s imperative, she said, that women are included at all stages across the lifespan, so researchers can understand how the female physiology responds to anything that is being studied. Salmerson said the NIH Office of Women in Research has resources available to researchers interested in studying women’s responses.
8. More research is needed
An overarching theme of the conference was the need for more research.
Government agencies are funding numerous projects focused on cannabidiol, THC and minor cannabinoids, seeking to understand how they work when used together and with conventional drugs.
The FDA’s National Center for Toxicological Research is also studying CBD in pre- and post-natal development, an ongoing, 2-3 year study looking at the neurobehavioral and neurochemical toxicity of purified CBD.
Laura Drotleff can be reached at [email protected]

